Storage & Handling1
Panzyga® can be stored for 36 months at 36°F
to 46°F (2°C to 8°C) from the date of manufacture
Within its shelf-life, panzyga® may be stored at room temperature (≤77°F or 25°C) for up to 12 months
- After storage at room temperature (≤77°F), product must be used or discarded
Do not freeze. Do not use frozen product
Do not use after expiration date
Does not contain preservatives
- Promptly use bottle after entering or opening
- Discard partially used bottles
Dispose of any unused product or waste material in accordance with local requirements
Components of packaging are not made with natural rubber latex
Do not mix with other products or administer with other preparations
Vial Sizes & NDC Numbers
Panzyga® is available in 5 vial sizes to help meet individual treatment needs1
Size : 2.5g / 25mL
NDC : 68982-822-82
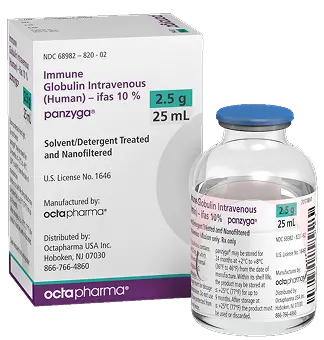
Size : 5g / 50mL
NDC : 68982-822-83
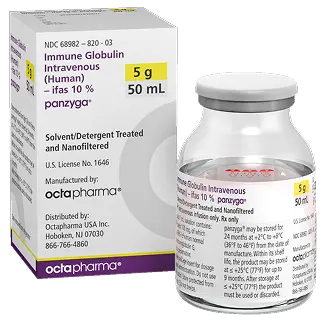
Size : 10g / 100mL
NDC : 68982-822-84
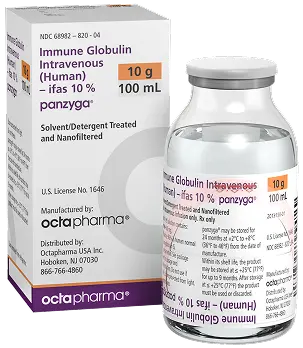
Size : 20g / 200mL
NDC : 68982-822-85
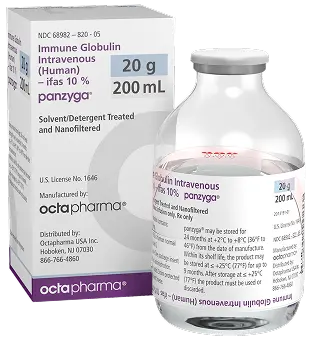
Size : 30g / 300mL
NDC : 68982-822-86
Code Description
J1576 Injection, immune globulin (panzyga®), intravenous, nonlyophilized (e.g., liquid), 500 mg
The information provided in this table is intended for informational purposes only and is not a comprehensive description of potential coding requirements for the product. Coding and coverage policies change periodically and often without warning. The healthcare provider is solely responsible for determining coverage and reimbursement parameters and accurate and appropriate coding for treatment of his/her patients. The information provided in this section should not be considered a guarantee of coverage or reimbursement for the product. The codes shown above are only general suggestions and are not intended to encourage or suggest a use of any drug that is inconsistent with FDA-approved use.
Overdosage1
With intravenous administration, overdose may lead to fluid overload and hyperviscosity. Patients at risk for complications of fluid overload and hyperviscosity include elderly patients and those with cardiac or renal impairment
Biochemical Composition & Manufacturing
Panzyga® is a solvent/detergent-treated, sterile preparation of highly purified immunoglobulin G (IgG)1
Panzyga® is a solution for infusion to be administered intravenously
Panzyga® is derived from large pools of human plasma
| Panzyga® is a stabilized with glycine and does not contain preservatives or sucrose1,2 | |
|---|---|
| Fractionation | Caprylic acid and chromatography |
| Total protein | 100 (≥96%IgG) |
| IgA (μg/mL) | Mean: 100 |
| Glycine (mg/mL) | 15.0 to 19.5 |
| Sodium (mmol/L) | Trace amounts |
| pH | 4.5 to 5.0 |
| Osmolality (mOsmol/kg) | 240 to 310 |
References: 1. Panzyga®. Prescribing information. Octapharma USA, Inc.; 2021. 2. Data on file. Octapharma USA, Inc.
Indications & Important Safety Information for panzyga® Immune Globulin Intravenous (Human) – IFAS 10% Liquid Preparation
Please click here for Full Prescribing Information, including BOXED WARNING.
- Thrombosis may occur with immune globulin intravenous (IVIg) products, including panzyga®. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients who receive IVIg products, including panzyga®. Patients predisposed to renal dysfunction include those with a degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IVIg products containing sucrose. Panzyga® does not contain sucrose.
- For patients at risk of thrombosis, renal dysfunction, or acute renal failure, administer panzyga® at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
See Full Prescribing Information, Warnings and Precautions (5.2, 5.4)
Indications and Usage
Panzyga® (Immune Globulin Intravenous [Human] – ifas) is indicated for the treatment of primary humoral immunodeficiency (PI) in patients 2 years of age and older; this includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies; chronic immune thrombocytopenia (cITP) in adults to raise platelet counts to control or prevent bleeding; and chronic inflammatory demyelinating polyneuropathy (CIDP) in adults to improve neuromuscular disability and impairment.
Contraindications
Panzyga® is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin and in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Warnings and Precautions
- Monitor renal function, including blood urea nitrogen and serum creatinine, and urine output in patients at risk of developing acute renal failure.
- Hyperproteinemia, increased serum osmolarity, and hyponatremia may occur in patients receiving panzyga®.
- Aseptic meningitis syndrome may occur in patients receiving panzyga®, especially with high doses or rapid infusion.
- Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to panzyga® treatments. Risk factors for hemolysis include high doses and non-O-blood group. Closely monitor patients for hemolysis and hemolytic anemia.
- Monitor patients for pulmonary adverse reactions (transfusion-related acute lung injury [TRALI]).
- Monitor blood pressure prior to, during, and following panzyga® infusion.
- Carefully consider the relative risks and benefits before prescribing the high dose regimen (for cITP) in patients at increased risk of volume overload.
- Panzyga® is made from human plasma and may contain infectious agents, e.g. viruses and theoretically, the Creutzfeldt-Jakob disease agent.
Adverse Reactions
- PI – The most common adverse reactions reported in greater than 5% of subjects were: headache, nausea, fever, fatigue, and abdominal pain.
- cITP in adults – The most common adverse reactions reported in greater than 5% of subjects were: headache, fever, nausea, vomiting, dizziness, and anemia.
- CIDP in adults – The most common adverse reactions reported in greater than 5% of subjects were: headache, fever, dermatitis, and blood pressure increase.
The risk information provided here is not comprehensive; See full Prescribing Information and Boxed Warning for panzyga®.
To report suspected adverse reactions, contact Octapharma USA, Inc. at 1-866-766-4860 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Indications & Important Safety Information for panzyga® Immune Globulin Intravenous (Human) – IFAS 10% Liquid Preparation
Please click here for Full Prescribing Information, including BOXED WARNING.
- Thrombosis may occur with immune globulin intravenous (IVIg) products, including panzyga®. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients who receive IVIg products, including panzyga®. Patients predisposed to renal dysfunction include those with a degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IVIg products containing sucrose. Panzyga® does not contain sucrose.
- For patients at risk of thrombosis, renal dysfunction, or acute renal failure, administer panzyga® at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
See Full Prescribing Information, Warnings and Precautions (5.2, 5.4)
Indications and Usage
Panzyga® (Immune Globulin Intravenous [Human] – ifas) is indicated for the treatment of primary humoral immunodeficiency (PI) in patients 2 years of age and older; this includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies; chronic immune thrombocytopenia (cITP) in adults to raise platelet counts to control or prevent bleeding; and chronic inflammatory demyelinating polyneuropathy (CIDP) in adults to improve neuromuscular disability and impairment.
Contraindications
Panzyga® is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin and in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Warnings and Precautions
- Monitor renal function, including blood urea nitrogen and serum creatinine, and urine output in patients at risk of developing acute renal failure.
- Hyperproteinemia, increased serum osmolarity, and hyponatremia may occur in patients receiving panzyga®.
- Aseptic meningitis syndrome may occur in patients receiving panzyga®, especially with high doses or rapid infusion.
- Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to panzyga® treatments. Risk factors for hemolysis include high doses and non-O-blood group. Closely monitor patients for hemolysis and hemolytic anemia.
- Monitor patients for pulmonary adverse reactions (transfusion-related acute lung injury [TRALI]).
- Monitor blood pressure prior to, during, and following panzyga® infusion.
- Carefully consider the relative risks and benefits before prescribing the high dose regimen (for cITP) in patients at increased risk of volume overload.
- Panzyga® is made from human plasma and may contain infectious agents, e.g. viruses and theoretically, the Creutzfeldt-Jakob disease agent.
Adverse Reactions
- PI – The most common adverse reactions reported in greater than 5% of subjects were: headache, nausea, fever, fatigue, and abdominal pain.
- cITP in adults – The most common adverse reactions reported in greater than 5% of subjects were: headache, fever, nausea, vomiting, dizziness, and anemia.
- CIDP in adults – The most common adverse reactions reported in greater than 5% of subjects were: headache, fever, dermatitis, and blood pressure increase.
The risk information provided here is not comprehensive; See full Prescribing Information and Boxed Warning for panzyga®.
To report suspected adverse reactions, contact Octapharma USA, Inc. at 1-866-766-4860 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.